ELICITEX For Chilli Growing
Benefits of Use for Chilli Growing
Defensive plant secondary metabolites (PSMs) not only improve disease resistance by boosting the plants innate (phytoanticipins) and adaptive immunity (phytoalexins) but also have beneficial effects on crop nutritional density, improve organoleptic properties including taste (flavour), smell (odour), colour, as well as shelf-life and customer satisfaction.
The pungency (strong heat) and piquancy ("agreeably stimulating to the palate") in chilli peppers is known to come from its secondary metabolite compounds known as capsaicinoids, especially capsaicin (8-methyl-N-vanillyl-6-nonenamide, typical relative amount 69%) and other related amide capsaicinoids including dihydrocapsaicin (typical relative amount 22%), nordihydrocapsaicin (typical relative amount 7%) present in the chilli pepper’s placental tissue (which holds the seeds), its internal membranes and to a lesser extent, the other fleshy parts of its fruit. The seeds themselves do not produce any capsaicin, although the highest concentration of capsaicin can be found in the white pith of the inner wall, where the seeds are attached.
The use of this strategy has been shown to increase the concentration of capsaicinoids and therefore increase the Scoville Heat Unit values of Capsicum plants.
Also, increased capsaicinoids levels increase resistance to Fusarium oxysporum and Fusarium solani which cause Fusarium wilt in chilli plants.
This strategy has been shown to be effective against Phytophthora capsici which is the cause of chilli phytophthora blight a dangerous disease in chilli peppers (plants belonging to the genus Capsicum) that may devastate crops unless properly controlled.
Scoville Organoleptic Test
Capsaicinoids are agonists of the transient receptor potential cation channel subfamily V member 1 (TRPV1), also known as the capsaicin receptor or the vanilloid receptor.
Capsaicinoids are ligands of the TRPV1 and are stereochemical complementarity to its active site and can bind to it and activate signal transduction which leads to burning sensation and pain (nociception).
TRPV1 is used by the mammalian somatosensory system. It is a nonselective cation channel that may be activated by a wide variety of exogenous and endogenous physical and chemical stimuli.
The best-known activators of TRPV1 are capsaicinoids, temperature greater than 43°C, acidic conditions and allyl isothiocyanate, the pungent compound in mustard and wasabi. Its endogenous activators include low pH (acidic conditions), the endocannabinoid anandamide, N-oleyl-dopamine, and N-arachidonoyl-dopamine.
TRPV1 receptors are found mainly in the nociceptive neurons of the peripheral nervous system, but they have also been described in many other tissues, including the central nervous system.
TRPV1 is involved in the transmission and modulation of nociception, as well as the integration of diverse painful stimuli. TRPV1 in the gut are important for the metabolic effects of capsaicin. Stimulation of TRPV1 receptors is known to bring about activation of the sympathetic nervous system (SNS) with the peripheral vasodilation. Capsaicin has been shown to increase fat burning in humans and animals through stimulation of the SNS and is known to stimulate uncoupling protein 1 (UCP1)-dependent thermogenesis.
The TRPV1 oral cavity receptors are located slightly below the surface in the mouth and mediate pungency and piquancy organoleptic properties when activated by lipophilic capsaicinoids.
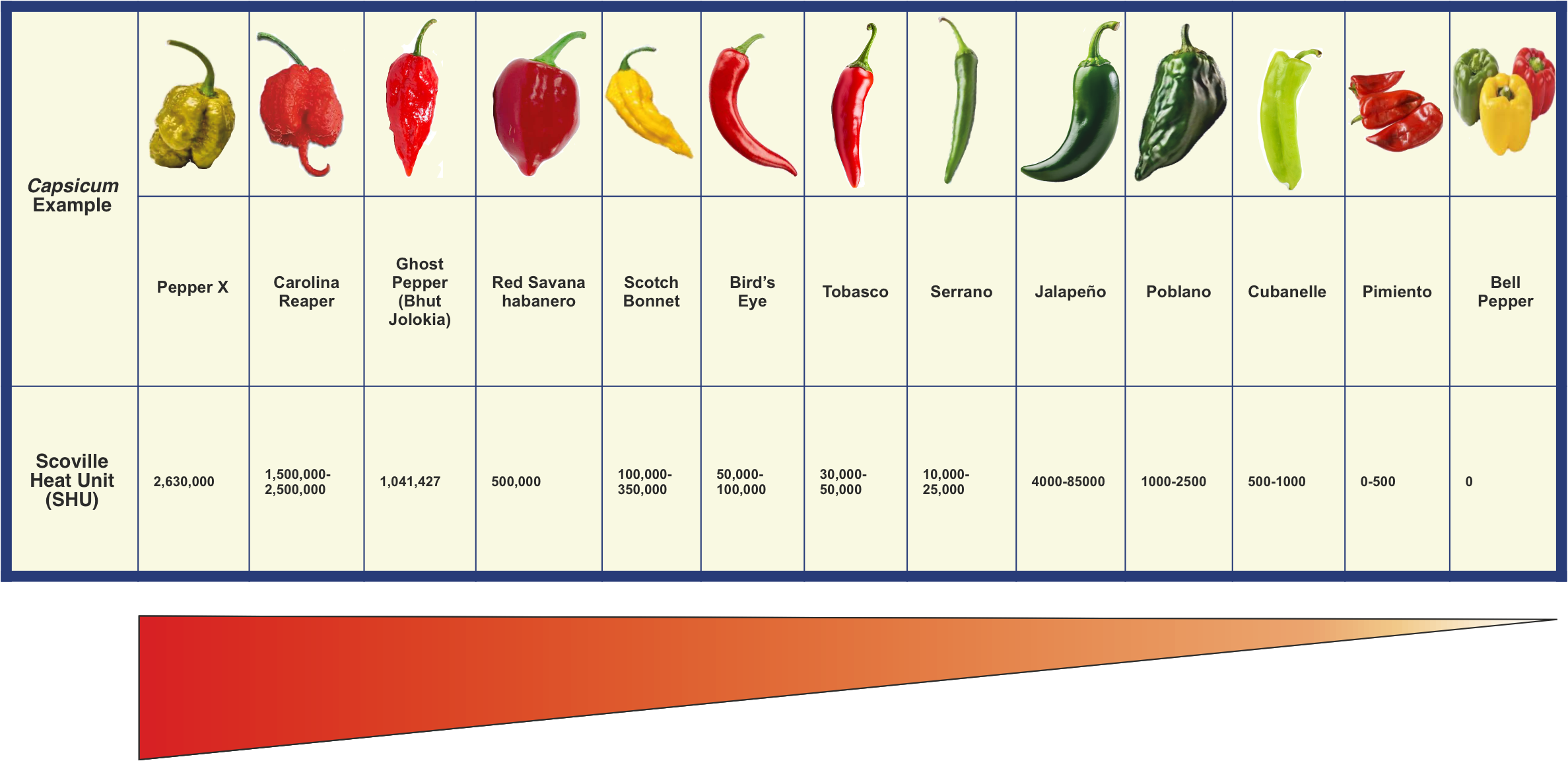
The Scoville Scale is a measurement of pungency (spiciness or "heat") of chilli peppers and other substances, recorded in Scoville Heat Units (SHU). It is based on the concentration of capsaicinoids and was the first laboratory test used to measure heat in chilli peppers.
The scale is named after its creator, American pharmacist Wilbur Lincoin Scoville, whose 1912 method is standardised and is known as the Scoville organoleptic test. The test is a subjective assessment based on the gustatory and olfactory capsaicinoid sensitivity of experienced tasters but it is limited by the variability of the tasters’ mouth heat receptors (TRPV1s) and the rapid desensitisation to capsaicinoids following multiple tastings (sensory fatigue) .
An exact weight of dried chilli is dissolved in alcohol to extract the heat components (capsaicinoids), then diluted in a sugar solution. Decreasing concentrations of the extracted capsaicinoids are given to a panel of five trained tasters, until a majority (at least three) can no longer detect the heat in a dilution i.e. the highest dilution of a chilli pepper extract at which heat can be detected by a taste panel. The heat level is based on this dilution, rated in multiples of 100 SHU.
The Scoville organoleptic test has been largely been replaced by chromatographic methods which are considered to be less subjective, more reliable and accurate.
Spice heat has been assessed by quantitative high-performance liquid chromatography (HPLC), which measures the concentration of heat-producing capsaicinoids.
This HPLC technique quantitatively analyzes capsaicinoids by measuring their concentration in parts per million (ppm) i.e. one part capsaicin equivalent per million parts dried pepper mass. This "parts per million of heat" (ppmH) is found with the following calculation:
ppmH = peak area (capsaicin) + 0.83 x peak area (dihydrocapsaicin)
peak area (standard)
Peak areas are determined from HPLC traces. The standard used to calibrate the calculation is 1g of capsaicin. The results are typically reported in ppmH (American Spice Trade Association (ASTA) pungency units), which can be converted to Scoville Heat Units (SHU) using a mathematical conversion factor, generally around 15 (ASTA Method 21.3: Pungency of Capsicums and Their Oleoresins).
There is a linear correlation between capsaicinoid concentration and the Scoville scale. Pure capsaicin has a Scoville scale rating of 16,000,000 SHU and pure dihydrocapsaicin has a Scoville scale rating of 15,000,000 SHU.
Carotenoids
Carotenoids are produced by all photosynthetic organisms and are primarily used as accessory pigments to chlorophyll in the light-harvesting part of photosynthesis.
They are highly unsaturated with conjugated double bonds, which enables carotenoids to absorb light of various wavelengths.
At the same time, the terminal groups regulate the polarity and properties within lipid membranes.
Most carotenoids are tetraterpenoids, regular C40 isoprenoids. Several modifications to these structures exist: including cyclization, varying degrees of saturation or unsaturation, and other functional groups.
Carotenes typically contain only carbon and hydrogen, i.e., they are hydrocarbons. Prominent members include α-carotene, β-carotene and lycopene, are known as carotenes.
Carotenoids containing oxygen include lutein and zeaxanthin. They are known as xanthophylls.
Their colour, ranging from pale yellow through bright orange to deep red, is directly related to their structure, especially the length of the conjugation.
Xanthophylls are often yellow, giving their class name. Carotenoids also participate in different types of cell signaling.
They are able to signal the production of abscisic acid, which regulates plant growth, seed dormancy, embryo maturation and germination, cell division and elongation, floral growth, and stress responses.
Chilli is a non-climatic fruit.
Non-climatic fruits are those that do not continue to ripen significantly after being harvested. Unlike climacteric fruits, which undergo a rise in respiration and ethylene production that triggers ripening (like bananas and tomatoes), non-climatic fruits reach their peak ripeness while still attached to the plant. Once picked, they do not improve in flavour or texture significantly.
Therefore, preharvest elicitation of chilli is important to accelerate the synthesis of PSMs involved in the ripening process and results in beneficial effects on crop nutritional density, improvements in organoleptic properties including taste (flavour), smell (odour), colour, as well as shelf-life and customer satisfaction.
The Catotenoid Biosynthetic Pathway
In plants, carotenoids perform a variety of roles associated with their potent antioxidant activity and characteristic chromophore, including accessory photosynthetic pigments, free radical scavengers and precursors of phytohormones such as abscisic acid and strigolactones.
Humans cannot synthesise carotenoids de novo; instead they must be acquired from the diet. The intake of fruit and vegetables rich in carotenoids has been shown from epidemiological and intervention studies to reduce the onset of chronic disease states, such as certain cancers, age-related macular degeneration and cardiovascular diseases.
Carotenoid pigments are isoprenoid molecules and thus derived from the universal five carbon precursor isopentenyl pyrophosphate (IPP, C5).
In higher plants, they are synthesised in the plastid using IPP generated from the methylerythritol-4-phosphate (MEP) pathway.
Geranylgeranyl pyrophosphate (GGPP, C20) is the prenyl lipid precursor utilised to form carotenes. Two molecules of GGPP are condensed in a head to tail manner by phytoene synthase. This reaction represents the first committed step in carotenoid formation.
Phytoene undergoes a series of sequential desaturation (dehydrogenation) steps to yield lycopene via phytofluene, ζ-carotene and neurosporene.
Carotene isomerisation, from cis to trans geometric configuration, is also an integral component of the sequence requiring two isomerases. All-trans lycopene can be cyclised to α-carotene and β-carotene via the action of ε- and/or β-ring cyclases.
In red tomato fruit, β-carotene is present but lycopene usually predominates. In contrast, the carotenoid pathway in Capsicum fruit extends to xanthophylls, particularly β-ionone ring-derived xanthophylls, unlike the ε-ring- (α-carotene) derived xanthophylls found in chloroplast-containing tissues.
The formation of xanthophylls in Capsicum species is initiated by the hydroxylation of β-carotene to form zeaxanthin. Epoxidation of zeaxanthin by the enzyme zeaxanthin epoxidase (ZEP) results in violaxanthin formation. This reaction sequence is reversible in nature through the action of violaxanthin de-epoxidase (VDE). Violaxanthin can also be converted to neoxanthin. The end-products found in red Capsicum fruit are the red pigments capsorubin and capsanthin, the latter being the most abundant. To form capsanthin/capsorubin, the action of capsanthin/capsorubin synthase (CCS) is required. The gene encoding this enzyme shows strong homology to carotene cyclase and it is postulated that the enzyme can also generate β-carotene in the fruit tissues. The CCS enzyme is responsible for generating the characteristic cyclopentane or κ-ring found in the Capsicum carotenoid pigments, capsanthin and capsorubin.
Phytoene synthase (PSY) is a key regulator in the biosynthesis of carotenoids and influences flux into the carotenoid pathway.
It is postulated that PSY expression is under the influence of pathway products and intermediates via feedback mechanisms.
Elicitex provides key biosynthetic enzyme substrates for PSY and increases gene expression of this enzyme transcript to significanty increase the synthesis of carotenoids.
Its activity is believed to be dependent on isoforms present and the intracellular location of these proteins.
In Arabidopsis, there is one isoform, tomato has three isoforms (leaf, fruit, and root), and pepper has been found to have two.
There have been reports of the PSY having differential levels of activity depending on its plastidial location.
Elicitation has been shown to increase gene expression of other enzyme transcripts including β-carotene hydroxylase (β-CH).
USE ON CHILLI
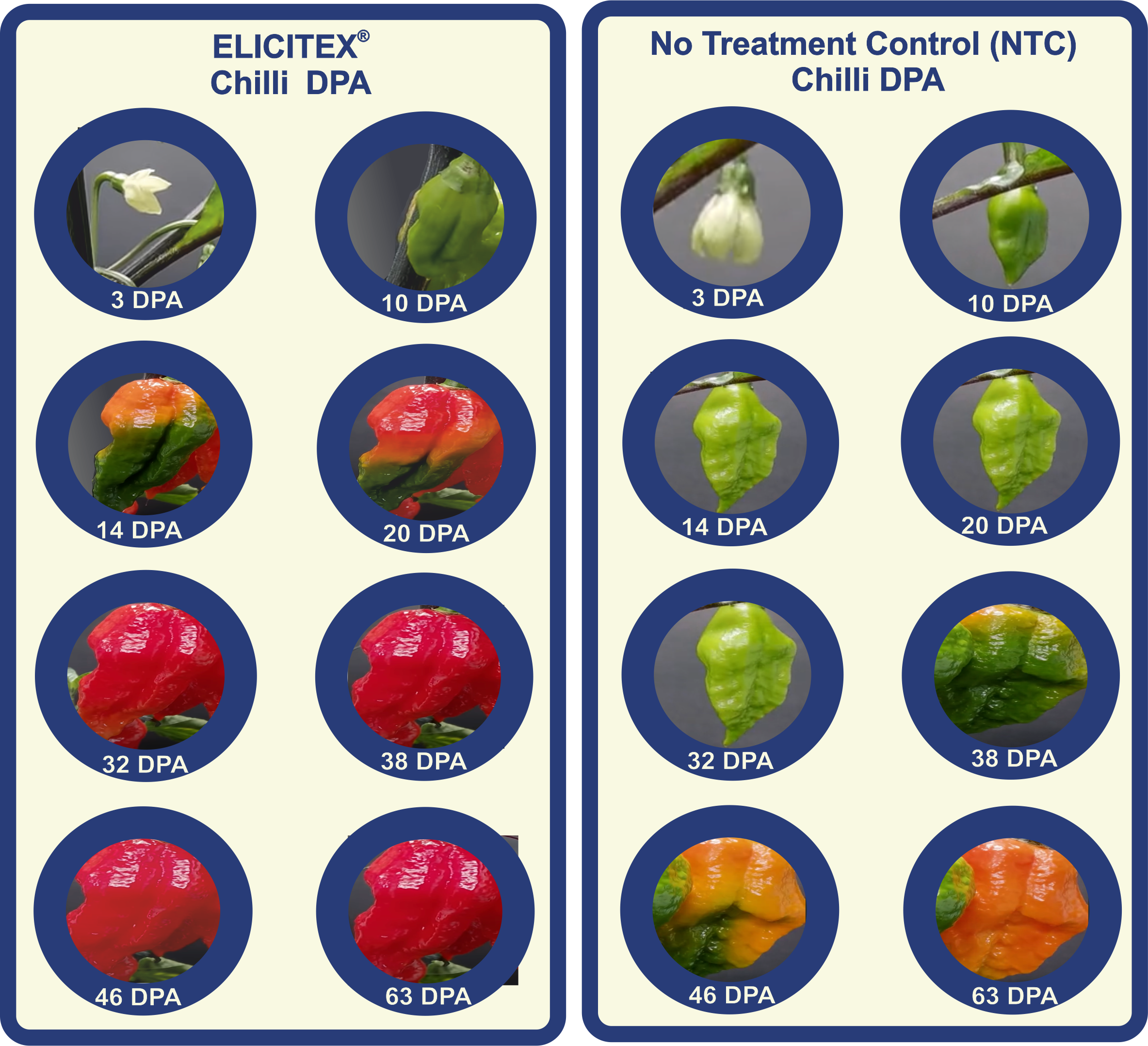
Elicitation of Capsicum assamicum, also known as ghost pepper, naga chilli or bhut jolokia (interspecific hybrid of Capsicum chinense and Capsicum frutensce), days post anthesis (DPA). Anthesis is the period during which a flower is fully open and functional.
Elicitex treatment induces rapid production of the red/yellow carotenoid pigments in chillies.
The Capsaicinoids Biosynthetic Pathway Through Phenylpropanoid and Branched-Chain Fatty Acid Pathways
The biosynthesis of capsaicin and dihydrocapsaicin involves convergence of two pathways, the phenylpropanoid and branched-chain fatty acid (BCFA) pathways.
Phenylalanine is produced from chorismate which is synthesised by the shikimate (shikimic acid) pathway in the plastid.
After its transport out of the plastid into the cytosol, it is converted into vanillylamine through a series of enzymatic reactions.
The other intermediate 8-methyl-6-nonanoyl-CoA is obtained from pyruvate through a series of steps and enzymatic reactions.
This involves the movement of valine into the mitochondria for its catabolism into isobutyryl-CoA and its re-entry back into the plastid.
After re-entering plastid, isobutyryl-CoA is elongated to 8-methyl-6-nonenoic acid by the enzymatic action of fatty-acyl-acyl carrier protein (ACP) thioesterase (FAT).
8-Methyl-6-nonenoic acid it is converted into 8-methyl-6-nonenyl-CoA by acyl-CoA synthetase (ACS) and is exported into the cytosol.
The final step involves the condensation of vanillylamine and 8-methyl-6-nonanoyl-CoA which is brought about by the enzymatic action of capsaicin synthase (CS), (also known as acyltransferase 3, AT3), which is encoded by the Pun1 gene and can produce different capsaicinoid derivatives based on the kind of BCFAs involved in the reaction.
MAVEN® TRIPLE P® and ELICITEX MIST® are registered trademarks owned by Maven International Limited
