ELICITEX MIST FAQs
![]()
PLANT DEFENCE BIOSTIMULATOR FOR ALL PLANTS
Here is a selection of some frequently asked questions about Elicitex Mist for your convenience.
General Directions
The TRIPLE P (Plant Promotion Products) range is formulated for the protection of professional continuous plant growth programmes in conjunction with Integrated Pest Management (IPM), Integrated Crop Management (ICM) and Biological Control Programmes. It is designed to deliver effective control of aphids, whiteflies, thrips and spider mites and good control of mealybugs, leafhoppers, psyllids, suckers and scale insects when applied as a contact penetration foliar spray. It also provides effective control of common root rot diseases, spread via zoosporic fungi such as pythium and phytophthora, when added to nutrient solution or irrigation water.
Question: What is ELICITEX MIST?
Answer: ELICITEX MIST is a proprietary mixture of modified plant elicitors in combination with a proprietary blend of optimised biostimulants. It contains a novel proprietary combination of food grade pathogen-associated molecular pattern (PAMP) elicitor polysaccharides and delivers highly effective environmentally sustainable protection from pathogenic fungi such as Alternaria solani and Phytophthora infestans (potato and tomato blight), Phytophthora capsici (pepper blight and rot), Alternaria cucumerina (cucurbit leaf blight in cucumber, watermelon and pumpkin), viruses and parasitic nematodes, as well as pest insect herbivores such as aphids, whiteflies, thrips, mealybugs, leafhoppers, psyllids, scale insects and archnid or mites such as spider mites. ELICITEX MIST is biocompatible, biodegradable and uses strategic green chemistry.
Question: How does ELICITEX MIST work?
Answer: ELICITEX MIST effectively generates and triggers multiple plant defence molecules inducing complex physiological and biochemical processes. Foliar application increases abscisic acid (ABA) content, increases stomatal conductance, reduces transpiration and significantly reduces water use without affecting plant height, root length, leaf area or plant biomass. An optimal blend of co-stimulatory elicitors has been combined with biostimulants, synergists, adjuvants and a delivery system for improved plant immunostimulation.
Question: Why does ELICITEX MIST contain elicitors and biostimulants?
Answer: Elicitors trigger endogenous plant chemical defence mechanisms by inducing complex physiological and biochemical processes. They do this by binding to specific receptor molecules on the plant surface which causes signal transduction to trigger the production of plant defence molecules and secondary metabolites.
This ELICITation requires EXtra biostimulation priming to induce effective activation of plant immunity.
A plant biostimulant is any substance or microorganism applied to plants which enhance nutrition efficiency, abiotic stress tolerance and/or crop quality traits, regardless of its nutrients content (du Jardin).
ELICITEX MIST is highly effective at inducing secondary metabolites and plant defence molecules.
ELICITEX MIST signals plants to use and divert some of its finite metabolic energy away from normal growth and development towards plant defence.
Question: What are the ELICITEX MIST benefits of use for chilli growing?
Answer: ELICITEX MIST improves disease resistance by boosting the plants innate and adaptive immunity, as well as having beneficial effects on crop nutritional density, improving organoleptic properties including taste (flavour), smell (odour), colour, as well as shelf-life and customer satisfaction. ELICITEX MIST has been shown to increase the concentration of capsaicinoids (pungency) and therefore increase the Scoville Heat Unit values (spiciness or "heat") of Capsicum chilli pepper plants. The use of this treatment induces rapid production of the red/yellow carotenoid pigments in chillies and has been shown to be effective against Phytophthora capsici which is the cause of chilli phytophthora blight, which is a dangerous disease in chilli peppers (plants belonging to the genus Capsicum) that may devastate crops unless properly controlled. Read about Elicitex for chilli growing to understand more on the benefits of growing chillies with ELICITEX MIST.
Question: What are the ELICITEX MIST benefits of use for soft fruit growing?
Answer: Soft fruits have considerable economic and nutritional value. Strawberries and raspberries are a rich source of beneficial bioactive plant secondary metabolites (PSMs). ELICITEX MIST has been shown to induce rapid and increased production of soft fruit plant secondary metabolites (PSMs) including the red anthocyanin pigments.
Strawberry, red raspberries and other soft fruits are susceptible to environmental factors and have a short shelf life (3–4 days).
ELICITEX MIST induces disease resistance in soft fruits by boosting the plants innate and adaptive immunity and inducing the production of plant secondary metabolites (PSMs) which also has beneficial effects on crop nutritional density, improvements in organoleptic properties including taste (flavour), smell (odour) and extended harvested crops shelf life. Read about Elicitex for soft fruit growing to understand more on the benefits of growing soft fruits with ELICITEX MIST.
Question: What are the ELICITEX MIST benefits of use for potato growing?
Answer: ELICITEX MIST induces disease resistance in Solanum tuberosum L. (potato) by boosting the plants innate and adaptive immunity, but also have beneficial effects on crop nutritional density, improvements in organoleptic properties including taste (flavour), smell (odour).
This strategy has been effective against Soft Rot Pectobacteriaceae (SRP) bacteria (Dickeya, Pectobacterium, and Musicola genera), which are prominent plant pathogens causing destructive soft rot diseases in potato. Read about Elicitex for potato growing to understand more on the benefits of growing potatoes with ELICITEX MIST.
Question: When and how to apply ELICITEX MIST to plants?
Answer: In order to use ELICITEX MIST correctly, it must be used from the late vegetative growth phase. If it is used in the early vegetative growth phase it may inhibit normal growth and development by signalling the plant to divert its energy into the production of defence molecules.
If too small a dose is used it will not exceed the no-observed effect level (NOEL). No plant defence molecules will be produced. If too large a dose is used, it will exceed the no-observed adverse effect level (NOAEL) and it will signal the plants to produce too many plant defence molecules at the expense of the production of the required molecules for normal growth and development. This will result in a decrease growth and toxicity. The maximum stimulatory dose occurs at the “M” dose. The NOEL, NOAEL and “M” dose will be different for different plant species and cultivars.
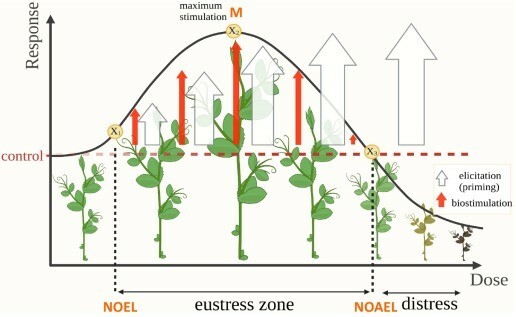
If the correct dose is used the plants will be in the eustress zone and will have improved growth and development and have improved immunity. They will be protected from predation, infection and produce secondary metabolites and molecules which enhance crop quality, flavour, nutritional benefit and post-harvest shelf life.
ELICITEX MIST is a ready to use spray and the dose can be adjusted in 2 ways. Firstly, by the frequency of spraying. We recommend spraying every 5 days. Secondly, the dose can be adjusted by regulating the spray nozzle (atomizer) to change the spray characteristics to a fine droplet mist (small sized droplets with higher number of drops/mL) from a spray jet. We recommend the use of a fine mist with even coverage and minimal run-off.
We also recommend that ELICITEX MIST is only applied to plants that are fully established in late vegetative phase and that plants are fully hydrated.
Question: What are the benefits of using ELICITEX MIST?
Answer:
- ELICITEX MIST provides fast and long acting indirect control of plant pathogens (by induction of endogenous defence Plant Secondary Metabolites)
- Fits well with Integrated Pest Management (IPM), Integrated Crop Management (ICM) and Biological Control Programmes.
- Acts as an anti-transpirant and significantly reduces water use (by abscisic acid induction and downstream effects on stomatal conductance).
- It is not harmful to beneficial organisms such as pollinators like bees or organisms which predate on plant pests.
- Active substances are food grade and can be used up to harvest without human toxicity.
- ELICITEX MIST induces disease resistance in Solanum tuberosum L. (potato) by boosting the plant innate and adaptive immunity. Active against potato Soft Rot Pectobacteriaceae (SRP) bacteria (Dickeya, Pectobacterium and Musicola genera). Elicits effective endogenous potato defence activity against the oomycete (omycota) Phytophtora infestans that causes late potato blight and the fungal pathogen Alternaria solani that causes early potato blight.
- Harvested crops have extended shelf lives, thus reducing food waste by delaying crop decay (improving food security).
- Improves soft fruit firmness and induces rapid production of the red anthocyanin pigments in strawberries.
- Rapid and highly efficacious induction of plant secondary metabolites (which include flavonoid anthocyanins, terpenes, phenolics, capsaicinoids, carotenoids and tocopherols) to increase nutritional density and improve organoleptic properties including flavour, smell, colour and customer satisfaction.
- Safe for use on all edible crops and degrades rapidly in the environment.
- Effective as a plant growth promoter, improves tolerance to osmotic, salinity, drought and extreme temperature stresses.
- Rapid and highly efficacious induction of secondary metabolites to improve crop quality, taste, phytochemical induction eg. capsinoids.
- Improves tolerances to osmotic, salinity, drought and extreme temperature stresses.
- Acts as a synergist with plant hormones, giving further growth boosting effects.
- Preconditioning of mother plants improves cloning success.
- It aids growing, regrowth, assists photosynthesis and sustains healthy-looking plants.
Question: What is Elicitation?
Answer: In plant biology, elicitors are extrinsic or foreign molecules often associated with plant pests, diseases or synergistic organisms. Elicitor molecules can attach to special receptor proteins located on plant cell membranes. These receptors are able to recognize the molecular pattern of elicitors and trigger intracellular defence signal transduction pathways such as the octadecanoid pathway. This response results in the enhanced synthesis of secondary metabolites which reduce damage and increase resistance to pest, disease or environmental stress. This is an immune response called pattern triggered immunity (PTI).
Plants produce a huge number of compounds that are not strictly necessary for growth and development, but which play a crucial role in defence and adaptation to the environment. These secondary metabolites, which include terpenes, steroids, phenolics, and alkaloids, exhibit a wide range of biological activities.
Plants serve as a source of nutrients for a wide variety of heterotrophic microorganisms that can cause diseases in their hosts. Physical barriers, such as a waxy cuticular layer and rigid cell walls, as well as preformed antimicrobial chemicals can provide some protection against attacking phytopathogens. In addition, plants have evolved an inducible immune system that is based on the specific recognition of pathogen-derived molecules.
Two classes of plant immune receptors are critical for defence activation. Pattern recognition receptors (PRRs) and effector-triggered immunity (ETI). PRRs directly interact with highly conserved microbe associated molecular patterns (MAMPs) activating pattern-triggered immunity (PTI). PTI can be attenuated or blocked by effector molecules that are secreted into plant cells by microbial pathogens that are well-adapted to their hosts. The remaining weakened host immunity operating during such compatible plant/pathogen interactions. A state also referred to as effector-triggered susceptibility (ETS) is called basal defence. While basal defence can limit the spread of virulent pathogens in their hosts, it is typically insufficient to prevent disease.
A second class of plant immune receptors, encoded by disease resistance (R)-genes, recognize the presence or activity of effectors and induce effector-triggered immunity (ETI), a manifestation of the well-described phenomenon of gene-for-gene resistance or race-specific resistance which leads to incompatible interactions. ETI is a strong immune response that efficiently protects plants from avirulent pathogens and is often associated with the hypersensitive reaction (HR), a form of programmed death of plant cells at infection sites. Purified molecules or crude biochemical preparations from pathogens triggering PTI have also been referred to as general elicitors, while those triggering ETI, or race-specific resistance, have been termed race-specific elicitors.
Numerous studies have shown that ETI, basal defence and PTI utilize a common set of signaling components including multiple regulatory proteins, reactive oxygen intermediates (ROIs) as well as the phytohormones salicylic acid (SA), ethylene (ET) and jasmonic acid (JA). Levels of ROI, SA, ET, or JA often increase in plant tissues after pathogen infections. While basal defence seems mainly to be a weakened form of PTI, ETI has been proposed to result from boosted basal defence or PTI associated responses. Inducible immune responses are tightly associated with extensive transcriptional and metabolism reprogramming controlled by a complex regulatory network.
While historically 10 classes of pathogenesis-related (PR) genes had been recognized, which exhibit transcriptional up-regulation in defence-related biological situations more recent genome-wide transcript profiling studies have revealed that 100–1000s of genes typically respond to defence induction by transiently altered transcript levels. Numerous signal transducers and transcription factors have been implicated in the plant defence network. This network can be subdivided into various defined sectors that can interact with each other.
For example, distinct defence signalling sectors dependent on early MAMP-activated MAP kinases (MAPKs) or the hormones SA or JA, have been described. Interestingly, some of these sectors were found to largely interact in an additive or synergistic fashion during PTI, while they are partially antagonistic to each other during ETI. The latter phenomenon seems to allow for compensatory effects if a defined sector is disabled due to interferences with pathogen effectors.
The complexity of this network is likely the result of two separate co-directional evolutionary pressures. Firstly, the asymmetrical arms race between plants and pathogens/pests manifested in continuous co-evolution of effectors and their host targets may have resulted in an ever-increasing diversity of plant defence regulators and regulatory circuits. Secondly, the need to fine-tune defence outputs appropriate for the respective attacker(s), which may exhibit biotrophic, hemibiotrophic, or nectrotrophic lifestyles, requires a complex regulatory system that allows for extensive crosstalk and compensatory interactions. An additional level of complexity likely arose from the need to link effector recognition mechanisms, which appear to be of recent evolutionary origin to more ancient regulatory processes mediating PTI.
While PTI, basal defence and ETI are transient local responses limited to pathogen infected tissues, plants can also activate long-lasting systemic immunity. Such systemic immunity can be initiated by local compatible or incompatible interactions resulting in systemic acquired resistance (SAR) or triggered by certain strains of non-pathogenic plant growth-promoting rhizobacteria (PGPR) leading to induced systemic resistance (ISR). SAR mediates long-lasting broad-spectrum resistance to a wide range of pathogens in uninfected tissues and organs. In addition to local pathogen infections, exogenous application of SA or SA analogs can induce SAR-like responses. SAR and related systemic immune responses have been demonstrated in several plant systems, such as cucumber, watermelon, tobacco, and Arabidopsis thaliana. Typically, SAR is associated with a local and systemic increase of SA levels that conditions enhanced expression of several classical PR genes. Some of these PR genes, such as PR1, PR2, and PR5 serve as robust markers for this systemic immune response.
While local and systemic accumulation of SA is critical for SAR induction, this hormone seems not to serve as a mobile signal mediating immunity in uninfected distal tissues. Several other small molecules have been proposed to fulfil such a role, such as methyl-salicylic acid (MeSA), azelaic acid, glycerol-3-phosphate, the abietane diterpenoid dehydroabietinal, JA, and the amino acid-derivative pipecolic acid. A central regulator of SAR is the transcriptional co-factor NON-EXPRESSOR OF PR GENES1 (NPR1). By interacting with TGA bZIP transcription factors, NPR1 seems to mediate up-regulation of the vast majority of SAR-associated genes. NPR1 activity has been proposed to be controlled by the SA-binding proteins NPR3 and NPR4, which can physically bind to NPR1 in a SA-concentration-dependent manner.
In contrast to SAR, induction of ISR is not associated with the accumulation of SA and PR transcripts. ISR has been shown to be triggered by the Pseudomonas fluorescens strain WCS417r and other non-pathogenic rhizobacteria in several plant species including Arabidopsis. In Arabidopsis, WCS417r-induced ISR acts against P. syringae pv. tomato, is dependent on JA and ET signalling, but does not require SA. Intriguingly, ISR is blocked in the Arabidopsis npr1 mutant. Thus, NPR1 also plays an important role in the ISR signaling pathway.
Upon perception of several exogenous defence-related stimuli, plants can establish an enhanced capacity to activate immune responses. This sensitization process, which is called priming, can be triggered by treatment of plants with necrotizing pathogens, beneficial microorganisms, wounding or with various natural and synthetic compounds. Once a pathogen infects primed plants, defence responses are activated faster and more robustly. Although this phenomenon has been known for years, its molecular basis is still only partly understood. Chromatin modifications, accumulation of dormant mitogen-activated protein kinases and alterations of primary metabolism have been shown to be associated with this process.
Elicitation is one the most effective techniques currently used for improving the biotechnological production of secondary metabolites. Elicitors are compounds that stimulate any type of plant defence, promoting secondary metabolism to protect the cell and the whole plant. According to their origin, elicitors can be divided into different types: (a) biotic and (b) abiotic. Abiotic elicitors can be considered as substances of non-biological origin, being predominantly inorganic compounds such as salts or physical factors. Inorganic chemicals like salts or metal ions have been used to increase the production of bioactive compounds by their modification of plant secondary metabolism. Salts (including AgNO3, AlCl3, CaCl2, CdCl2, CoCl2, CuCl2, HgCl2, KCl, MgSO4, NiSO4, VOSO4 and Zn ions) can elicit PSM production in a variety of plant species in culture systems such as cell suspensions, hairy roots and adventitious roots.
The majority of biotic elicitors are recognized by specific receptors bound to the cell membrane. This stimulus is then transferred to the cell by a signal transduction system, producing changes that ultimately lead to the formation of phytoalexins. Phytoalexins are antimicrobial substances, some of which are antioxidative as well. They are defined, not by their having any particular chemical structure or character, but by the fact that they are defensively synthesized de novo by plants that produce the compounds rapidly at sites of pathogen infection. In general, phytoalexins are broad spectrum inhibitors. They are chemically diverse and different chemical classes of compounds are characteristic of particular plant taxa. Phytoalexins tend to fall into several chemical classes, including terpenoids, glycosteroids and alkaloids. However, the term applies to any phytochemicals that are induced by microbial infection.
The response of the plant is determined by several factors, principally its genetic characteristics and physiological state. In general, plant resistance to disease is controlled by plant resistance (R) and pathogen avirulence (Avr) genes. However, while specific Avr products trigger defence responses in cultivars with matching R genes, the action of general elicitors can activate defences in cultivars of more than one species.
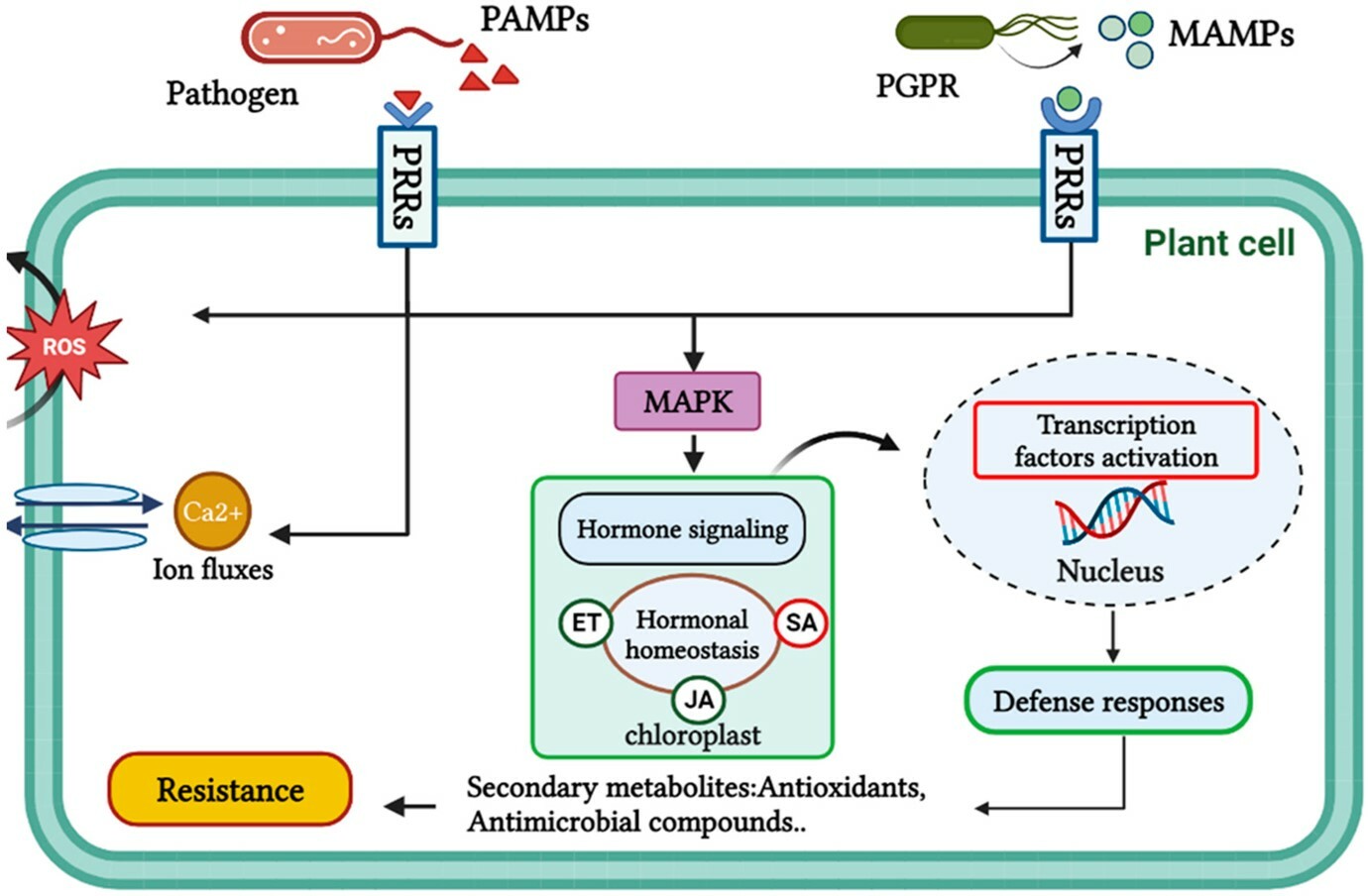
SCHEMATIC 8. Schematic representation showing the activation processes of plant defence responses. These defences are triggered when a stimulus is perceived by PRRs located on the surface of plant cells. Pathogens and PGPR are both capable of inducing the production of molecular signals that activate hormonal signalling pathways in plants. Specifically, ROS, calcium, and MAPK are among the key molecular signals that are increased in response to these microorganisms, ultimately leading to the development of plant resistance.
Question: What is Chitin and Chitosan?
Answer: Chitin and chitosan are typical molecular signatures for whole classes of microbes, which are known as microbe-associated molecular patterns (MAMPs) or pathogen-associated molecular patterns (PAMPs) that elicit plant immunity to control diseases. Additionally, chitin and chitosan could also promote plant growth and induce various physiological responses in plant against abiotic stress. The bioactivities of chitin and chitosan are closely dependent on their structure factors. Molecular size is the main factor affecting the bioactivity of homogeneous chitin. In contrast, chitosan, as a heterogeneous and cationic polysaccharide, is much more complex. Because commercially available chitosan is produced by chemical deacetylation of chitin under heterogeneous conditions, it usually results in chitosan products with random sequences. It mainly contains homogeneous GlcN oligomers and different GlcN/GlcNAc sequences. Some minor chitin oligosaccharides (GlcNAc) are also supposed to be present in a chitosan chain. Chitosan could be classified by molecular weight (MW), degree of polymerization (DP), degree of acetylation (DA), and pattern of acetylation (PA).
This heterogeneity greatly affects the physicochemical properties of chitosan, thus governing its biological applications. Therefore, which fragments are responsible for the observed biological effects of chitin and chitosan is a matter of continuing research. In recent years, with the rapid development of separation and purification technology, more and more chitin and chitosan fragments with well-defined structures are available and used for bioassays to understand the biological roles of chitin and chitosan.
Chitin is found as a major structural component of the fungal cell wall. Plants do not contain chitin but could efficiently digest it by chitinases that degrade chitin into oligosaccharide fragments. These chitin fragments could be recognized as stress signals by plant cells and elicit defense reactions. As a well-documented plant elicitor, chitin fragments have been reported to induce a set of defense reactions in many plant species, including depolarization of membrane potential, phytoalexin synthesis, defence gene induction, and enzyme activation.
The elicitor activity of chitin closely depends on its molecular size and degree of acetylation. It has been reported that chitin oligosaccharides larger than pentaose strongly induced the formation of phytoalexins in suspension-cultured rice cells at a very low concentration (10–9-10–6 M). In contrast, those chitin fragments smaller than trimer were inactive and a series of deacetylated chitosan oligomers also showed negligible elicitor activity. Similarly, Takai et al. reported that the expression level of an elicitor-responsive gene (EL5) was enhanced by addition of chitin heptamer or octamer, whereas chitin hexamer or shorter oligosaccharides or nonacetylated oligosaccharides showed much weaker activity. Additionally, Cord-Landwehr et al. prepared some partially deacetylated chitosan oligomers with a specific acetylation pattern GlcNAc-GlcNAc-(GlcN)n-GlcNAc (n ≥1) by chitin fragments deacetylation using fungus chitin deacetylases and found these specific chitosan oligomers products no longer elicited an oxidative burst in rice cell suspension culture. Such strict requirements for the size and structure of chitin oligosaccharides strongly indicate the presence of specific recognition and a signal transduction system for these oligosaccharides in plant cells.
Chitin is a well-known and conserved PAMP in the field of plant immunity. In the past decades, much pioneering work on identifying the high-affinity binding protein of chitin fragments has been performed to make clear the molecular mechanisms for plants recognizing and transducing the signals of chitin oligosaccharides. The cell surface receptor chitin elicitor receptor kinase 1 of Arabidopsis (AtCERK1) directly binds chitin through its lysine motif (LysM)-containing ectodomain (AtCERK1-ECD) to activate immune responses. The crystal structure of an AtCERK1-ECD complexed with chitin fragments was solved in 2012, revealing that chitin octamer, but not tetramer or pentamer, can act as a bivalent ligand to induce AtCERK1-ECD dimerization that is critical for its activation.
The molecular basis of the specific recognition of chitin oligosaccharides was also identified in rice (Oryza sativa), which clarified the formation and activation of the receptor CEBiP (chitin-elicitor binding protein) complex. Furthermore, Hayafune et al. proposed a model of unique sandwich-type dimerization of the rice receptor CEBiP activating chitin-induced immune signaling as illustrated in SCHEMATIC 9. It is demonstrated that the LysM1 domain of CEBiP accommodates chitin fragments in a hydrophobic cleft that hosts four GlcNAc moieties. Two of these four GlcNAc residues mainly interact through hydrogen bonding and hydrophobic interactions mediated by their acetyl groups.
Because outer ends of oligosaccharides barely contribute to binding, chitin hexamer is speculated to be the shortest oligosaccharide to saturate the four binding subsites in the LysM1 cleft. These biochemical data offer rational explanation for the aforementioned elicitor activity varying considerably among different chitin fragments, with the highest activity for longer-chain heptamers and octamers and little or no activity for tetramers and pentamers. In addition, the elicitor activity is also sequence specific. It is reported that the alternately N-acetylated oligosaccharides (GlcNβ1,4GlcNAc)4, carrying N-acetyl moieties only on one side, was unable to induce CEBiP receptor dimerization but inhibited the reactive oxygen species (ROS) generation induced by the chitin octamer, suggesting that the presence of alternating N-acetyl groups in (GlcNβ1,4GlcNAc)4 enables the fragment to recognize the binding site of CEBiP but could not trigger dimerization and activation processes (SCHEMATIC 9.).
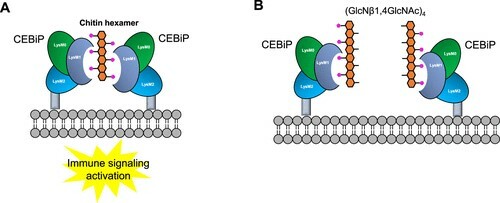
SCHEMATIC 9. A model of the activation of CEBiP complex by chitin fragments, proposed by Hayafune et al. (A) “Sandwich” model of activation of OsCEBiP receptor complex by chitin fragments. (B) Hypothetical model of dimerization and activation (oxidative burst) inhibition by (GlcNβ1,4GlcNAc)4.
As mentioned above, some papers reported that neither fully or partially deacetylated chitosan fragments could trigger oxidative burst and activation of the receptor CEBiP in a rice cell and thus were considered to have no elicitor activity. However, numerous studies have proved chitosan induced various physiological and biochemical responses to disease control in many plants, including cereals, ornamental, fruit, and medicinal plants. Induction of natural defence response by chitosan includes increased accumulation of phenolic compounds, cell wall synthesis, ROS generation, and so on.
Chitosan and its fragments can be used to enhance the plant’s defence against bacteria, fungi, virus, and nematodes. These works provide strong evidence for chitosan as a potent elicitor. Compared with chitin oligomers, chitosan oligomers are actually easier to be prepared at a large scale and is more suitable for practical application in the field of agriculture. The effectiveness of chitosan as an elicitor is also significantly correlated with its chemical structure. Degree of polymerization, DA, PA, and sequences are all important structure factors. So far, there are only a few reports on identifying chitosan fragments responsible for their elicitor activity. Hadwiger et al. prepared several chitosan oligomers by stereo controlled synthesis and verified the octamer optimally induced pisatin accumulation in peas and inhibited fungal growth.
Recently, some well-defined chitosan fragments with specific sequences were used to figure out the structural effect on elicitor activity of chitosan. Gubaeva et al. compared the ability of various chitosan oligomers with different DA and sequences to elicit an oxidative burst indicative of induced defence reactions in Arabidopsis thaliana seedlings. They found the elicitor activity of chitosan oligomers increased with increasing DA. Partially acetylated chitosan fragments required a minimum DP of 6 and at least four N-acetyl groups to trigger a response. On the other hand, Basa et al. proposed a hypothesis that small chitosan fragments are priming-active but elicitor-inactive substances, like other bacterial exopolysaccharides and alga sulphated polysaccharide.
Besides elicitor activity, PAMPs can also trigger plant cells to enter a state of elevated alertness that enables plants to react more quickly and more strongly following the detection of a pathogen, known as priming. The priming activity of chitosan was also found to be sequence specific. A monoacetylated chitosan tetramer was observed to possess the highest priming activity when the GlcNAc residue was located at the nonreducing end (ADDD). Furthermore, a deacetylated GlcN residue at the reducing end also seemed to be necessary for priming activity. Other partially acetylated tetramers with a GlcN residue at the reducing end (DADD, AADD) were also priming-active, but neither fully nor partially deacetylated tetramers with an acetyl group at or close to the reducing end (DDDD, DDDA, DDAD, DAAA, ADAA, AADA) induced priming. The structural complexity of chitosan causes great difficulty in its mode of action though much work has been done. Some important downstream signal pathways, including Ca2+, nitric oxide (NO), phytohormone (jasmonic acid, salicylic acid, and abscisic acid), were found to be involved in the chitosan transduction network.
However, the recognition receptor of chitosan in plants has not yet been elucidated clearly. Liu et al. demonstrated an interaction of fluorescence-labelled chitosan fragments with wheat leaf cell and further purified a chitosan binding protein from wheat plasma membrane proteins, but they did not report the function of this chitosan binding protein. So far, whether the specific receptors of chitosan exist or not still remains controversial. The cationic property of chitosan was also considered as an important aspect for the binding of chitosan with plant cell. It is still a challenging task to clarify the structure–function relationship and action mode of complex chitosan elicitors.
Chitosan and its fragments have also attracted increasing attention as a plant protection agent, effectively inducing plants against abiotic stress, such as water deficit, salinity, heavy metal toxicity, and chilling stress.
These unfavourable environmental conditions commonly cause many deleterious effects on plant growth, which mainly include the production of ROS, lipid peroxidation of membrane, inhibition of nutrients, and water uptake due to low external osmotic potential or the toxic effect resulting from higher ions accumulation. Corresponding to these physical changes in plants under stress conditions, antioxidant enzymes, malondialdehyde (MDA) concentration, proline content, and photosynthetic parameters were generally applied to evaluate the protective effect of chitosan on plants against abiotic stress. When treated with chitosan, the plants had lower MDA content, higher proline content, increase of chlorophyll content, and enhanced antioxidant enzymes such as superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) activities, indicating that chitosan could to some extent induce resistance against adversity stress.
Zong et al. conducted a hydroponic pot experiment to study the roles of chitosan with different MW (10 kDa, 5 kDa, and 1 kDa) in alleviating heavy metal Cd toxicity in edible rape (Brassica rapa L.). It is reported that spraying chitosan oligomers onto the leaves of edible rape under Cd-toxicity could decrease the proportion of Cd in the organelle fraction of leaves while increasing the proportion of Cd in the soluble fraction, suggesting that the protective effect of chitosan is partially attributed to Cd sequestration into the vacuoles, thereby reducing the availability and toxicity of Cd for rape leaf cells. In addition, the alleviation effect of chitosan on toxicity was found to be dependent on its MW, and the chitosan oligomers with a MW of 1 kDa showed the optimal activity.
Similar results were reported in studies on chitosan enhancing salt tolerance of wheat seedlings. In order to further make clear the structure–function relationship of chitosan affecting plant growth under abiotic stress, we conducted plant treatment using a series of fully deacetylated chitosan fragments with a defined size (chitotetraose to chitooctaose, DP8-10, and DP10-12). The application of chitosan fragments with different sizes in wheat seedlings under salt stress obviously decreased the MDA concentration, increased the contents of chlorophyll and proline, promoted the photosynthesis, activated antioxidant enzyme activities (SOD, POD, and CAT), and induced the expression of salt-related genes (SOS1 and NHX2). Chitohexaose, chitoheptaose, and chitooctaose were found to have the best activity, which are supposed to be the key components of chitosan inducing plant against the adverse effect of salt stress. In terms of plant suffering from chilling stress, Zou et al. reported chitohexaose and chitoheptaose showed the most effective activities alleviating the negative effects among six fully deacetylated single chitosan oligomers (chitobiose to chitoheptaose), while those short-chain chitosan fragments (dimer and trimer) produce little or no stimulation effect on MDA and proline contents.
Another issue to be noted is that these fully deacetylated chitosan oligomers may not to be the best fragments responsible for its activity inducing plant against various adversity stresses. The activity was also closely related to the DA of chitosan. It has been reported a chitosan oligomer mixture with intermediate DA had more effective activities in promoting wheat seedlings tolerance to salt stress than those with other DA.
Question: What is Biostimulation?
Answer: A plant biostimulant is any substance or microorganism applied to plants with the aim to enhance nutrition efficiency, abiotic stress tolerance, or crop quality traits, regardless of its nutrient content. The role of biostimulants is to control and accelerate the life processes of plants, increase the resistance to stress, and stimulate their development. These products are also safe for the environment and contribute to sustainable, high-output low-input crop production. Their application helps to reduce the amount of chemicals used in agriculture. Among biostimulants, plant extracts, amino acids, and beneficial elements have been widely studied.
Biostimulants based on proteins or amino acids have enhanced N metabolism, crop yield, grain characteristics and the content of macro- and micronutrients in winter wheat. As discussed, in plant science, the phenomenon of “priming” or elicitation has been investigated and reported frequently. It consists of applying low doses of a stressor, making the plants activate their defence system at a high and long-lasting level, and remain “conditioned” in the face of future stress situations. In addition, the concept of priming includes that the progeny, as one of its characteristics, could inherit this “super immunity” phenotype. Priming or elicitation is a phenomenon that favours channelling the energy of the plant towards the induction of the defence system, thus causing energy expenditure or sacrifice for the growth and development of the plant.
In addition to this, a relatively new type of agrochemicals known as “biostimulants” has become currently used in agricultural practices because they enhance the growth and development of plants. By definition, these biostimulants are products of biological origin of variable composition that, in low doses, favour phenotypes towards the growth and development of plants. It is also known that some of these biostimulants, when used in higher doses, commonly show a significant increase in tolerance towards biotic or abiotic stressors (i.e., there is an activation of plant immunity, that is, priming or elicitation as mentioned above), which is associated with lower growth and development and some of them can even be toxic for plants in higher doses. This latest evidence suggests that some biostimulants might induce hormesis in some evaluated variables.Hormesis is a two-phased dose-response relationship to an environmental agent whereby low-dose amounts have a beneficial effect and high-dose amounts are either inhibitory to function or toxic.
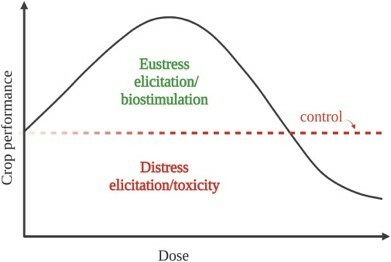
Therefore, it can be hypothesized that a biostimulant could improve phenotypical variables (i.e., growth and development) and/or activate immunity sacrificing growth and development (i.e., they cause elicitation or priming) in a dose-dependent manner while provoking toxicity at even higher doses, that is, causing distress. In agriculture, biostimulation and elicitation or priming generates phenotypes that can be considered favourable within a specific range depending on the variable that motivates crop production (these phenotypes fall within the eustress zone in a hormetic curve (Schematic 13.).
It is noteworthy that according to du Jardin (2015), the definition of a biostimulant includes only biological stressors. However, both chemical and physical stressors can also show hormetic responses in the eustress zone in plants, suggesting that the biostimulant concept should be reviewed based on current experimental evidence. Therefore, based on a potential scenario of hormesis when applying a biological, chemical, or physical stressor to plants, it is reasonable to expect eustressing effects, depending on the dose and the variable evaluated. Distressing/toxic scenarios could also occur at higher doses.


Schematic 13. Crop biostimulation and elicitation (priming) in the context of the hormesis model. The maximum stimulatory dose occurs at the “M” dose, while X1 and X2 represent the NOEL (No-Observed Effect Level) and NOAEL (No-Observed Adverse Effect Level), respectively. The eustress zone is characterized by the stimulation of growth and productivity (biostimulation) and simultaneous activation of defence mechanisms (elicitation). If the NOAEL is exceeded, biostimulation ceases due to hormetic trade-offs, but plant defence increase to toxic levels.
A specific plant response depends on the stressor applied and the response variable measured. Plants respond in such a way that ultra-low stressor doses do not generate an observable phenotypic response (i.e. No-Observed-Effect-Level, NOEL). Still, once this dose level is exceeded, the treatment can stimulate response variables until reaching a Maximum Stimulation dose (“M” dose), then the responses diminish until a dose that generates toxicity once the so-called No-Observed-Adverse-Effect-Level (NOAEL) is passed.
Depending on its dose, a stressor might trigger in plants stimulant features such as increasing growth, yield, production quality (biostimulation), increase stress tolerance (elicitation), or toxic phenotypes. When a stressor triggers a stimulant response, a positive stress or eustress occurs (i.e., the stressor is an “eustressor”). On the contrary, if the response is toxic, either deleterious or lethal, it is called negative stress or distress (i.e., the stressor is a “distressor”)
By definition, these biostimulants are products of biological origin of variable composition that, in low doses, favour phenotypes towards the growth and development of plants. It is also known that some of these biostimulants, when used in higher doses, commonly show a significant increase in tolerance towards biotic or abiotic stressors (i.e., there is an activation of plant immunity, that is, priming or elicitation as mentioned above), which is associated with lower growth and development. Likewise, it is also possible to find non-hormetic responses for variables in dose-response studies. Multiphasic responses to allelopathic or allelochemical compounds that depend on the duration of the stress application or the evaluated variable have been reported.
Thus, depending on the dose, these compounds can significantly improve the physiology of the plants for growth and development (biostimulant) and strengthen their immune systems (elicitor) in such a way that the plants display a eustress behaviour. Biostimulants are viable without having to apply them in the quantities usually used of toxic synthetic agrochemicals, thus producing results with minimal levels of toxicity or even without detected toxicity to the environment or living beings. Given the increasing demands on biomass for food and feed purposes, the importance of biologically active substances, often referred to as biostimulants, is emphasized. According to Regulation (EU) 2019/1009 of the European Parliament and of the Council
MAVEN® TRIPLE P® and ELICITEX MIST® are registered trademarks owned by Maven International Limited
